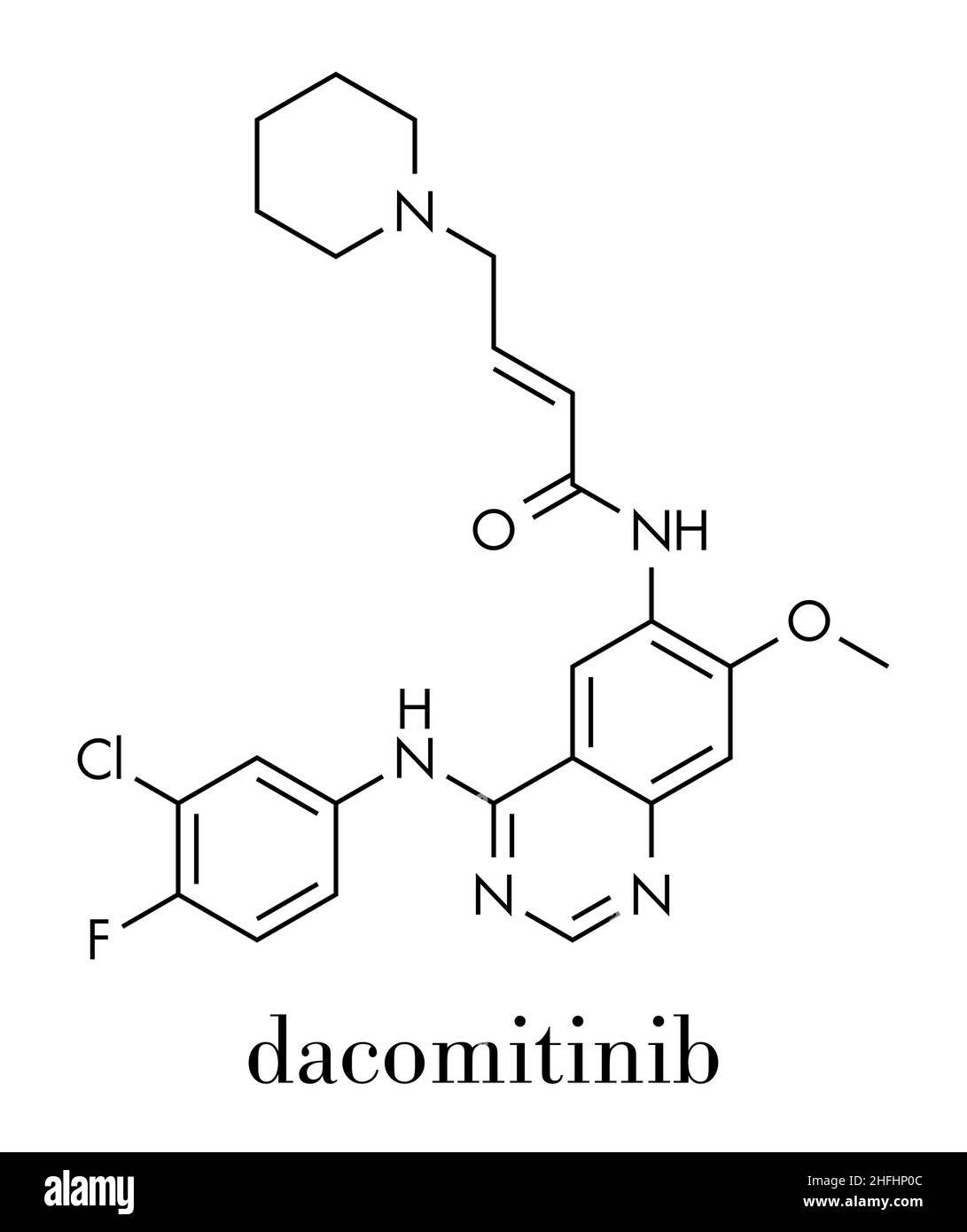
Dacomitinib, sold under the brand name Vizimpro, is a medication for the treatment of non-small-cell lung carcinoma (NSCLC). It is a selective and irreversible inhibitor of EGFR.
Dacomitinib has advanced to several Phase III clinical trials. In January 2014, results of the first trials were disappointing, with a failure to meet the study goals, but additional Phase III trials continued. In 2017, results of a trial comparing dacomitinib to gefitinib for NSCLC (driven by mutated EGFR) were announced.
Dacomitinib was approved for medical use in the United States in September 2018, in Japan in 2019, and in the European Union in 2019; for the treatment of non-small cell lung cancer with epidermal growth factor receptor (EGFR) gene mutation.
References
External links
- "Dacomitinib". Drug Information Portal. U.S. National Library of Medicine.